UP Chemists Modify Anticancer Compound to Improve Safety and Efficacy
Published: January 23, 2024
By: Harvey L. Sapigao
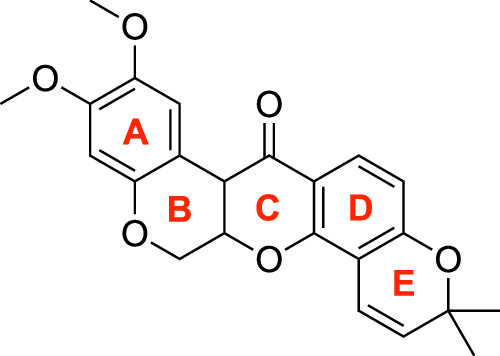
Scientists from the University of the Philippines – Diliman College of Science (UPD-CS) have transformed the anticancer compound deguelin into a novel class of compounds that show promise as safer and more effective treatments for colon, lung, and breast cancer.
Originally used as a pesticide and fish poison, concerns arose regarding the safety of administering deguelin to humans once it was identified as a cancer treatment. Science Research Specialist John Alfon Francisco and Dr. Monissa Paderes of the UPD-CS Institute of Chemistry (UPD-CS IC) addressed these concerns by altering the structure of deguelin.
Their altered versions exhibited better qualities than the original compound. Preliminary tests done on human cancer cell cultures revealed reduced adverse effects, prompting Dr. Paderes and collaborators to conduct further research into the safety of these compounds.
Some versions are also more effective against specific types of cancer. “We were amused to find that some compounds have improved anticancer properties than its parent compound, deguelin, with some even surpassing the effectiveness of the commercially available anticancer drug doxorubicin,” Francisco said. For instance, a version named 6a outperformed doxorubicin in treating colon cancer, while versions 3a and 8e excelled in treating lung and breast cancer, respectively.
Their modified versions offer a simpler and more cost-effective production than those developed in previous studies. “The simplicity of the structures, as well as the straightforward synthesis of these compounds, add to the novelty of this study,” Dr. Paderes emphasized.
The researchers created the altered versions by shortening a part of deguelin known as the BCE ring, making the new versions more akin to the deoxybenzoin compound, recognized for its antibacterial and antioxidant properties.
Despite its huge commercial potential, their research is still in its early stages. The next phase involves testing the modified anticancer compounds on animal models. If the compounds are proven effective on animals, it will move on to clinical trials, where it will be tested on humans with colon, lung, or breast cancer. If proven successful, the Food and Drug Administration (FDA) will review and approve the rollout of the compounds as cancer treatments.
“The goal would be to advance these compounds toward clinical trials and potential development as novel anticancer therapeutics,” Dr. Paderes concluded.
For interview requests and other concerns, please contact media@science.upd.edu.ph.
References:
Francisco, J. A., & Paderes, M. C. (2023). Inhibitory effects of B-, C-, and E-ring-truncated deguelin derivatives against A549, HCT116, and MCF-7 Cancer Cells. ACS Omega, 8(45), 43109–43117. https://doi.org/10.1021/acsomega.3c06619
